It’s safe to say that photochemistry is having a moment. Whether you’re working in pharma, agrochemicals or materials science, the impact that harnessing photochemistry could have upon your research is significant. However, the use of photochemical reactors is often avoided due to perceived high costs, safety worries, imprecise temperature control, inconsistencies between reactor designs, limitations with the wavelengths of light available for a particular apparatus, irreproducible results and the significant length of time required to run a series of experiments.
This short article will showcase Asynt’s Illumin8 Parallel Photoreactor, an exciting new plug-and-play innovation that addresses all of the drawbacks outlined above, and mounts easily and effectively upon a standard hotplate stirrer.

Photochemistry: providing access to novel chemical transformations
Although the concept of photochemistry has been established since the 19th century, with some continuation of work through the 20th century, the past decade in particular has seen significant advances in the use of photochemically-mediated chemical transformations, for example through late-stage heterocyclic metamorphosis, modification of polymer-based hydrogels, C(sp2)-H amination and decarboxylative azidation of cyclic amino- and tertiary-acids, to name a few.
In particular, photoredox catalysis has emerged as a powerful tool within synthetic organic chemistry. Commercially available photocatalysts are derived from a range of metals, including ruthenium, iridium, copper and tungsten, and the wavelengths of light available range from UV to visible to NIR, allowing fine-tuning of transformations and tailoring a reaction to a particular need.
There are many benefits afforded using photoredox catalysis, including:
- Mild reaction conditions that allow late-stage functionalisation and therefore rapid structure derivatisation and library generation;
- Novel disconnections not readily available through thermally-mediated reactions can be sought;
- A continually growing array of commercially available photoredox catalysts, allowing bespoke tuning of catalytic properties;
- Highly reactive intermediates, or entirely new modes of reactivity, can be accessed in situ;
- Wavelengths of light can be tuned to induce excitement of a single chromophore, allowing for precise reaction engineering for example in the functionalisation of biomacromolecules.
Asynt and Partners: Illumin8-ing the way
Asynt have teamed up with Sygnature Discovery, and Liverpool ChiroChem (LCC), to showcase their Illumin8 parallel photoreactor. In particular, both companies noted that the ability to run reactions in parallel, the accurate temperature control, and the facile interchange of LED light sources allowed versatility of the set-up and rapid generation of late-stage compound libraries for further screening, which was a significant advantage over other commercially available systems.
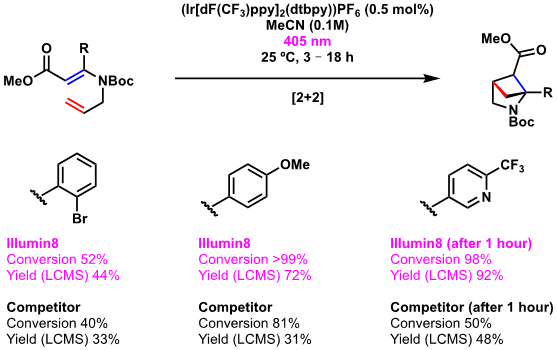
[2+2] reactions exploiting iridium-mediated photoredox catalysis
The pyrrolidine motif is commonplace within medicinal chemistry and drug discovery. Increasing the rigidity of the structure by installing additional sp3 character through a bridging methylene unit has been shown to improve DMPK profiles of numerous drugs and drug candidates, as well as improve selectivity, therefore decreasing off-target activity. In this case, Sygnature Discovery used the Illumin8 parallel photoreactor to complete a range of [2+2] cycloadditions.
Electron-rich and electron-poor R groups were tolerated (Scheme 1 illustration), and in all cases the performance of the Illumin8 parallel photoreactor was superior to competitor products both in terms of starting material conversion and LCMS-determined yields.
Scheme 1: Iridium-mediated [2+2] cycloaddition to form a bridged pyrrolidine
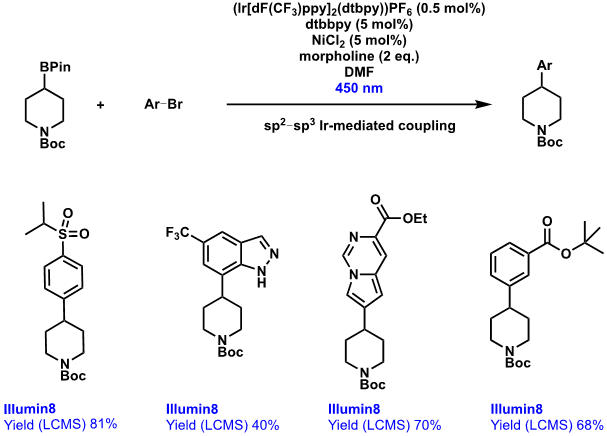
C(sp2)–C(sp3) cross-couplings (without palladium)
Another area in which iridium-based photocatalysis has provided a valuable alternative to more established palladium-based techniques is for C(sp2)–C(sp3) couplings between aryl bromides and pincacolborane species. The Suzuki reaction is known to lead to unwanted side-reactions, such as proto-dehalogenation, and can be beleaguered by sluggish reaction rates, neither of which are ideal when at the end of a 10-step synthesis! The use of photoredox catalysis allows for low catalyst loadings and a valuable alternative coupling approach, with only simple filtration followed by solvent removal required before preparative purification.
A broad array of aryl groups were tolerated (Scheme 2 illustration), containing a range of functionality.
![]()
Scheme 2: Iridium-mediated C(sp2)–C(sp3) coupling reactions to form aryl-substituted piperidines.
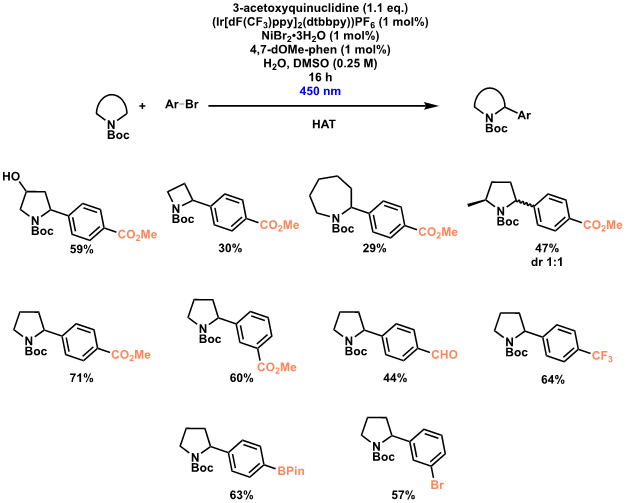
Photoredox-mediated hydrogen atom transfer (HAT)
Photoredox-mediated hydrogen atom transfer (HAT) in combination with nickel catalysis allows for C–H activation/functionalisation at the α-position of N-heterocycles. Our partners LCC, using a procedure initially disclosed by MacMillan, rapidly synthesised a library of α-substituted N-heterocycles, with a range of aryl bromides and N-heterocyclic ring sizes tolerated.
Initial experiments used similar lamps to those by MacMillan, but use of the Illumin8 Parallel Photoreactor allowed for rapid reaction optimisation and synthesis of a range of compounds due to the possibility of completing eight reactions in parallel. In addition, the excellent temperature control and the facile switching of LEDs to test varying wavelengths was highly appreciated (Scheme 3 illustration).

Scheme 3: Iridium-mediated α-functionalisation of N-heterocycles.
“The Illumin8 allowed us to rapidly screen substrates in an often-sensitive reaction and provide consistent results.
Using the equipment, we were able to produce a wide range of novel building blocks and improve upon some of our existing routes.”
Ross Goodyear, Deputy Team Leader, Liverpool ChiroChem
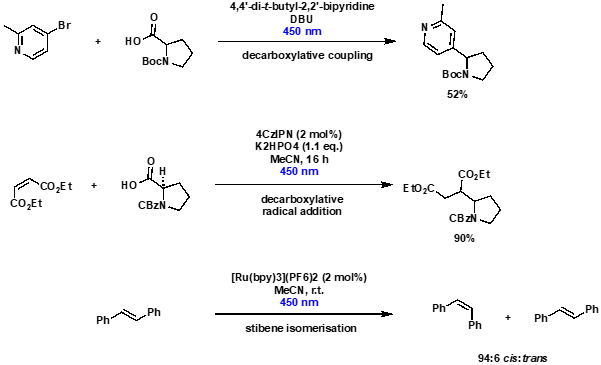
The Illumin8 for radical couplings and alkene isomerisation
As well as state-of-the-art photoredox reactions, the Illumin8 Parallel Photoreactor has also been used in more established transformations, including radical-mediated decarboxylative coupling, decarboxylative radical addition and alkene isomerisation, Scheme 4. In particular, the accurate temperature control ensured that stilbene isomerisation occurred at room temperature (22 °C), whereas use of an alternative commercially available photochemistry system resulted in reaction heating, and a less favourable cis:trans ratio of isomers.
Scheme 4: Use of the Illumin8 Parallel Photoreactor to undertake radical-mediated decarboxylative coupling, decarboxylative radical addition and alkene isomerisation.
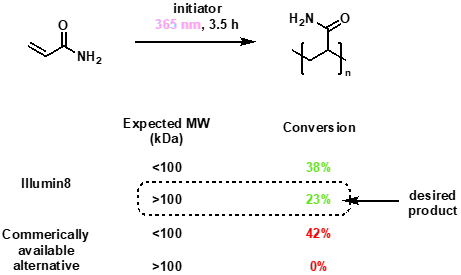
Significant control over polymerisation side-reactions
Our final example showcases how the Illumin8 Parallel Photoreactor has been used to prepare polyacrylamide from the acrylamide monomer. A comparison was made between a commercially available product and the Illumin8 system operating with LEDs at 365 nm. The desired product had molecular weight <100 kDa and was not observed with the commercially available lamp but was a significant component during irradiation under the Illlumin8 system. In addition, the Illumin8 system maintained the temperature at 28 °C, even after 3.5 hours of irradiation, whereas the other commercially available system resulted in significant reaction warming.
Scheme 5: Use of the Illumin8 Parallel Photoreactor to polymerise acrylamide.
The Illumin8 Parallel Photoreactor, a key addition to your research laboratory
The Illumin8 Parallel Photoreactor has been successfully used across a range of organic reactions, including under photoredox conditions with transition metal catalysts, more ‘traditional’ radical chemistry couplings, alkene isomerisation and radical-mediated polymerisation. Importantly, the ease with which a library could be generated, the precise temperature control and ability to screen a range of LED wavelengths meant that our partners could rapidly and reliably screen conditions, saving time and improving efficiency.
In summary, the Illuminn8 Parallel Photoreactor provides numerous advantages when compared with a conventional photochemistry system, including:
- Running up to eight reactions in parallel, from 1 mL to 8 mL, allowing rapid generation of compound libraries;
- Excellent temperature control due to the fan cooling system, with temperatures between –30 and 80 °C reliably accessible when using the heating module;
- The lock-feature prohibiting LED activation unless the unit is completely closed, giving unparalleled safety credentials;
- Rapidly interchangeability of the LED modules, with nine wavelengths of light available between 365 nm (UVA) and 660 nm (NIR);
- Efficient stirring due to the unit mounting quickly and easily onto a hotplate stirrer;
- Facile sample collection for TLC analysis and the ability to degas experiments, as well as the possibility of running experiments under positive gas pressure due to the reaction tubes being topped with septa.
Talk to us about your photochemistry research requirements
To see how the Illumin8 Parallel Photoreactor can help you with your research endeavours, please reach out to our team today and book an appointment or a demonstration. You can call +44 (0) 1638 781709 (UK) or 502-593-0726 (USA), email [email protected], use the live chat here on our website, or click HERE to book an online 1-2-1 video meeting.