 Chiral alpha-halo ketones derived from N-protected amino acids are key building blocks for the synthesis of HIV protease inhibitors such as atazanavir used in HAART combination therapy.
Chiral alpha-halo ketones derived from N-protected amino acids are key building blocks for the synthesis of HIV protease inhibitors such as atazanavir used in HAART combination therapy.
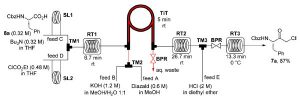
Kappe and De Souza have reported a continuous flow through route to these intermediates which utilises a tube-in-tube reactor to introduce diazomethane generated on demand into the reaction stream containing mixed anhydride derivatives of N-protected amino acids. The resulting alpha-diazo ketones are then decomposed with HCl or HBr to afford the corresponding alpha-halo ketones.
This process allows the safe generation, separation and use of diazomethane in a continuous integrated multi-step synthesis of important API intermediates.




