Novel laboratory apparatus specialists, Asynt, are pleased to share that the Cresswell Group, led by Dr Alexander J. Cresswell at the University of Bath (UK), have demonstrated the synthesis of a number of spirocyclic tetrahydonaphthyridines (THNs) expanding the chemical space of these important scaffolds for drug discovery. Presented at the recent Automated Synthesis Forum in Oxford, UK, and supported by promising unpublished work, the group are using a combination of photochemistry with flow chemistry to achieve significant results.
This work shows a novel photoredox-catalysed hydroaminoalkylation (HAA) of halogenated vinyl pyridines, followed by either an intramolecular N-arylation via SNAr, or by a palladium-catalysed C-N bond formation in continuous flow to enable an automated synthesis of α-alkylated and spirocyclic 1,2,3,4-tetrahydro-1,8-naphthyridines (“1,8-THNs”).
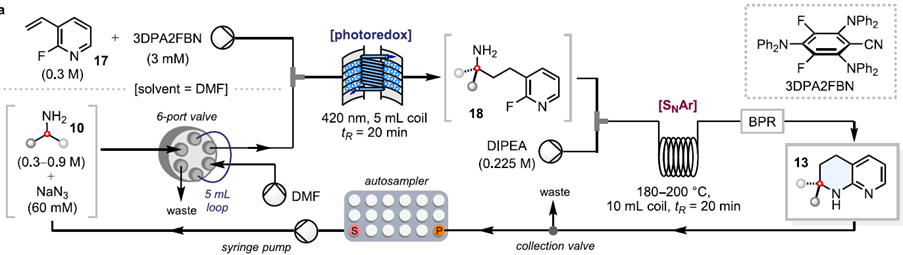
The key step in the synthesis, the photocatalysed reaction (HAA), was performed using the Uniqsis PhotoSyn from Asynt. This high-power LED photoreactor lamp module for continuous flow applications is equipped with a high performance 420nm LED array (716 individual diodes) which was mounted on a Cold Coil with a 5 mL PFA Coil and cooled to -0.50C using a Huber CC-805 chiller. The ability to maintain a low temperature with a light intensity of 350 W at 420 nm was a key factor in the success of this reaction, capabilities that only the PhotoSyn possesses when compared with other market solutions.
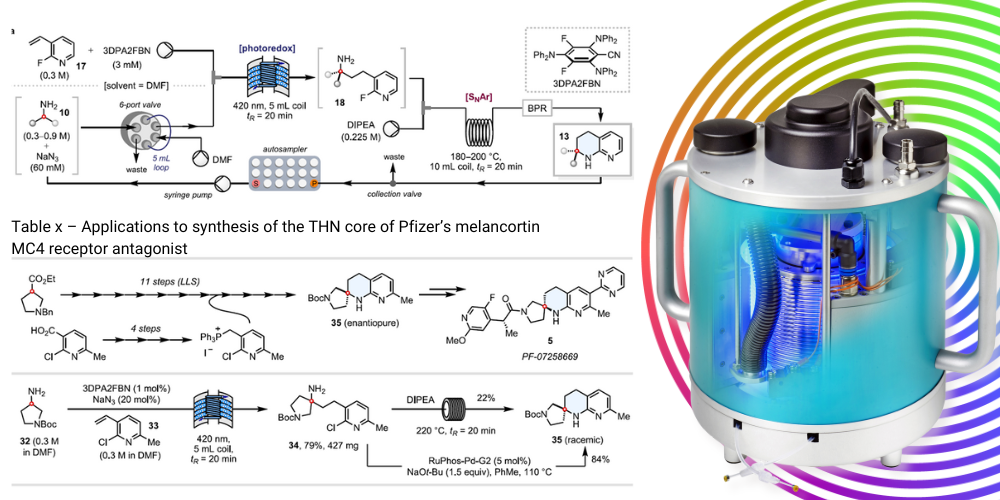
Automated photo-flow synthesis with PhotoSyn
Tetrahydronaphthyridines (THNs) are semi-saturated bicycles that ring-fuse a piperidine with a pyridine, two of the most popular N-heterocycles used in medicinal chemistry. These bicyclic compounds feature saturated N-heterocycles fused to (hetero)aromatic units offering eight different structural isomers through the positioning of the two THN nitrogen atoms. These structural motifs can be found in the MC4R antagonist PF-07258669 in phase 1 clinical trials from Pifzer and in the development of the FGFR4 selective inhibitor Roblitinib from Novartis. THNs and their isomers can be used in a number of biosteres increasing their importance.
As well as demonstrating the reaction optimisation leading to the automated continuous flow synthesis of THNs from primary amines, the group – using an autosampler – showed both a library synthesis and that the process is applicable for a range amines, including cyclic amines and multiple functional groups – all with good to excellent yields.
Expanding this work further, the researchers applied their methodology to a concise synthesis of the spirocyclic THN core of Pfizer’s MC4R antagonist, previously synthesised in 15 steps. The industrial route, whilst 11 steps in the longest linear sequence, is enantioselective, compared to a racemic synthesis in this case. Nevertheless, this illustrates how dramatically the synthesis of complex spirocyclic amines can be streamlined when using a photoredox annulation strategy from unprotected amines.
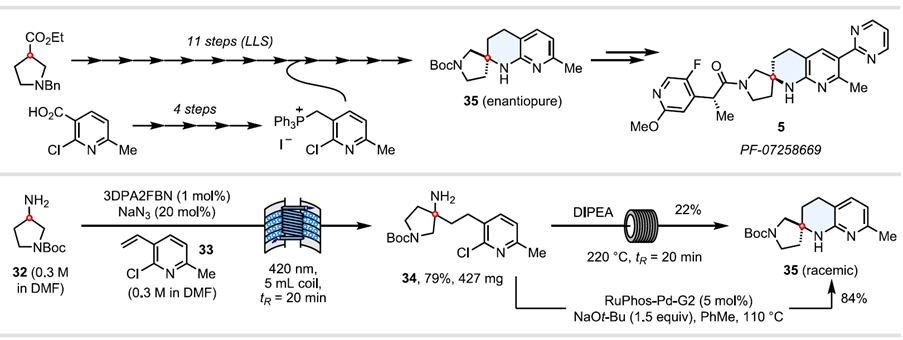
Table x – Applications to synthesis of the THN core of Pfizer’s melancortin MC4 receptor antagonist
Conclusion
In summary, the researchers have developed an automated, continuous flow synthesis of α-alkylated and spirocyclic 1,2,3,4-tetrahydro-1,8-naphthyridines (“1,8-THNs”), in addition to their regioisomeric 1,6-THN analogues, from abundant primary amine feedstocks.
The key step in this work was the photocatalysed HAA reaction step which utilises the unique design of the Uniqsis PhotoSyn photochemical reactor from Asynt, engaging both the high power and low temperatures which were essential for the success of the reaction.
Further information
For further information on the PhotoSyn photochemical reactor please visit https://www.asynt.com/product/photosyn/.
To find out more about the Cresswell Group, please visit their website at https://cresswell-lab.wixsite.com/cresswellgroup.
Reference
Cao, Q., Tibbetts, J.D., Wrigley, G.L. et al. Modular, automated synthesis of spirocyclic tetrahydronaphthyridines from primary alkylamines. Commun Chem 6, 215 (2023). https://doi.org/10.1038/s42004-023-01012-2







